Irrigation water use is water artificially applied to farm orchard pasture and horticultural crops as well as water used to irrigate pastures for frost and freeze protection chemical application crop cooling harvesting and for the leaching of salts from the crop root zone. Water is everywhere from huge oceans to invisible water molecules making up water vapor in the air.

Why Does Water Support Life Overview Examples Expii
Water can be used for direct and indirect purposes.

. Pure liquid water at room temperature is odorless tasteless and nearly colorless. Describe the properties of water as it is heated from ice to liquid water to a gas. Describe the unique properties of water.
Direct purposes include bathing drinking and cooking while examples of indirect purposes are the use of water in processing wood to make paper and in producing steel for automobiles. The bulk of the worlds water use is for agriculture industry and electricity. Water enables nutrients proteins amino acids glucose and other compounds to be consumed and assimilated by the body.
Disadvantages of Water as a Solvent. Water is highly cohesive and adhesive. This is called adhesion.
The specific enthalpy of fusion of water is 33355 kJkg1 at 0 C. Surface tension heat of vaporization and vapor pressure. Science Chemistry QA Library Describe how the properties of water in relation to IMFs contribute to the upward movement of water in a tree.
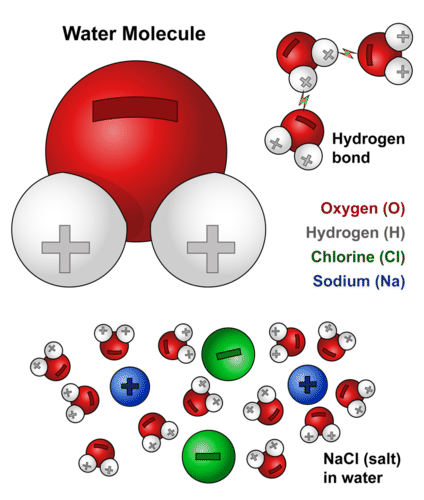
Distinguish between cohesion and adhesion of water molecules and describe the capillary action. Water is an excellent solvent and therefore it helps in the transportation of ions and molecules required for metabolism. Because of hydrogen bonds water molecules develop strong intermolecular.
Fill in the missing ions in the acid- base reaction of a carbonate ion with water below. List the properties of water. Water molecules are attracted to other molecules that contain a full charge like an ion a partial charge or polar.
Be sure to include an explanation of the filtration systems we use to keep water safe for drinking. Poisons are soluble in water. Water dissolves sugar salt and other flavourings.
If there is too much water on the Earth the salts will dissolve and sink deeper. Drinking preparing food bathing washing clothes and dishes brushing your teeth watering the garden and even washing the dog. Describe how the properties of water in relation to IMFs contribute to the upward movement of water in a tree.
Use the quantities constant low medium and high to describe the terms. This is due to cohesion of water molecules. Due to this property small organism float or.
This property causes water molecules to be drawn to one another. In our body water brings nutrients to all the cells and oxygen to our brain. The Many Uses of Water.
Water has a faint blue color which becomes more apparent in large volumes of water. These properties form the reason for its significance in the biosphere. Water is made up of polar molecules so polarity is one of its unique properties.
The soil then contains an insufficient amount of minerals. This can be used to flavour dishes. More on the latter in the.
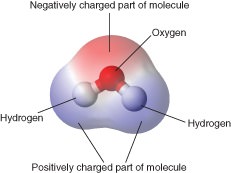
The hydrogen atoms are positively charged and the oxygen molecules are negatively charged. The cat doesnt dip its tongue to. Because water seems so ubiquitous many people are unaware of the unusual and unique properties of water including.
There is also the ability of water to bind with molecules of different substances. Water can form hydrogen bonds which make it a powerful solvent. High Specific Heat.
Boiling and freezing points. Toxins and waste are washed out by sewage. Water manages to stay liquid because of two of its properties high specific heat and its high heat of vaporization.
Each water molecule has two hydrogen atoms and one oxygen atom H2O. Of course you can see and feel the physical properties of water but there are also many chemical electrical and atomic-scale properties of water that affect all life and substances on Earth. Describe the preparation properties and uses of some representative metal carbonates Question Carbonate ions can be converted to bicarbonate ions by an acid-base reaction with water.
Describe how the properties of water allow cats to drink in this fashion including how waters molecular structure contribute to this process 2 See answers Advertisement Advertisement meerkat18 meerkat18 The way a cat drinks liquid is credited to the two forces that they have to balance. The unique physical properties including a high heat of vaporization strong surface tension high specific heat and nearly universal solvent properties of water are also due to hydrogen bonding. Water is found everywhere in different forms.
Water is a polar molecule that has a high level of polarity and attraction to ions and other polar molecules. Water has high tension. Domestic water use is water used for indoor and outdoor household purposes all the things you do at home.
Nonagricultural activities include self-supplied water to irrigate public and private golf courses parks. As compared to other liquids water has a higher specific heat thermal conductivity surface tension dipole moment etc. These molecules form polar covalent bonds.
It is a good conductor of electricity and has different properties. It is cohesive and adhesive It has a high specific heat It has a high heat of vaporization It is less dense as a solid than a liquid It is a good solvent 5. Under ordinary conditions of temperature and pressure water exists as a atom vaporization which is the ability to cling to other polar liquid Water also exhibits surfaces because of its polarity cohesion electronegative Water has a high heat which allows it to absorb heat without greatly changing its temperature and a high heat of which prevents it from easily becoming a gas and.
Water is colourless odourless and tasteless. Apart from these there are various other properties that make water unique. Be sure to include the terms density mass volume and expand.
Describe the hydrogen-bond between water molecules. Make sure the equation is balanced. Water is the lightest in gas form whereas a liquid is much heavier than its solid form.
Water has a simple molecular structure. Through this property water can be adhesive to any other molecule it can form a hydrogen bond with. Properties of Water and its Importance to Life 1.
Water has the second highest specific enthalpy of fusion of all substances after ammonia.

Lesson Summary Water And Life Article Khan Academy

Properties Of Water Physical Chemical Properties Chemistry

What Are The Unique Properties Of Water

Why Does Water Support Life Overview Examples Expii
Biochemical Properties Of Water Advanced Read Biology Ck 12 Foundation

The Structure And Properties Of Water Introduction To Chemistry
Biochemical Properties Of Water Advanced Read Biology Ck 12 Foundation

The Structure And Properties Of Water Introduction To Chemistry

0 Comments